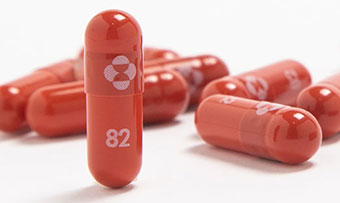
Molnupiravir
Of the participants who received molnupiravir 28. Molnupiravir FDA Approval Status.
A novel coronavirus originally identified in Wuhan City China was reported to the World Health Organization on 31 December 2019 and the associated disease has subsequently become a worldwide pandemic.

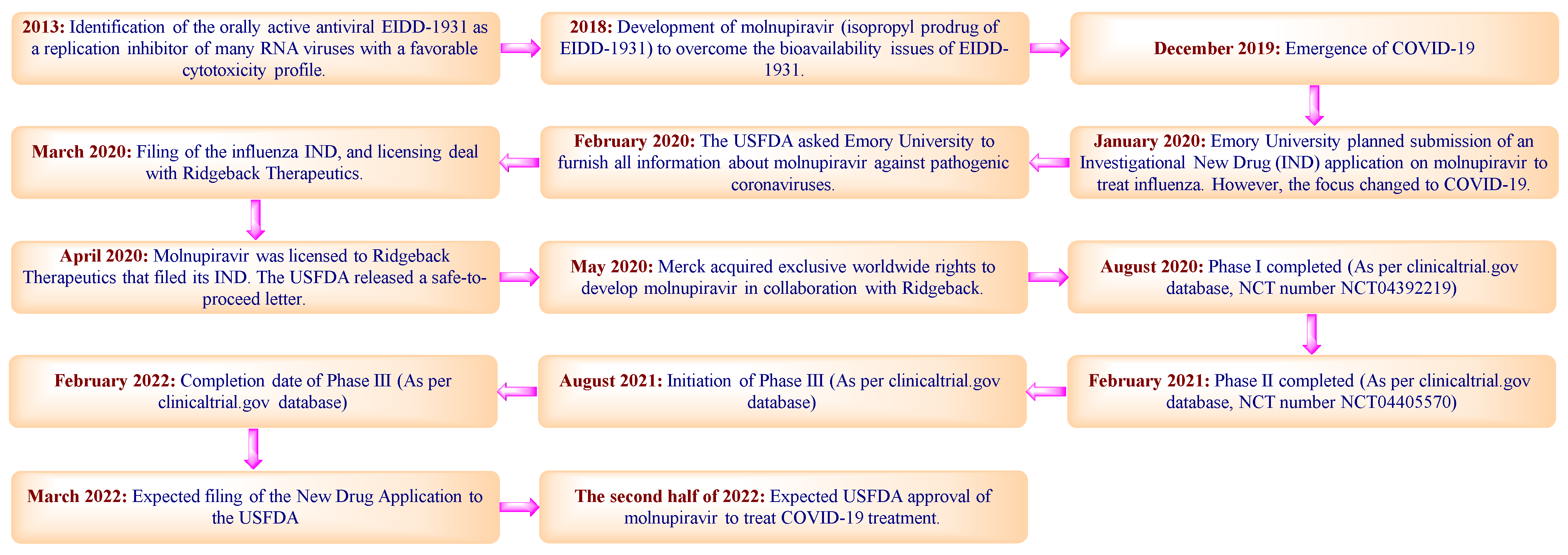
Molnupiravir. Molnupiravir ist ein experimentelles Virustatikum das ursprünglich zur Behandlung von Infektionen durch Influenza- und Coronaviren entwickelt wurde. Molnupiravir has also been shown to be active in several preclinical models of SARS-CoV-2 including for prophylaxis treatment and prevention of transmission. In March 2021 the companies reported preliminary results from a Phase IIa trial of molnupiravir for Covid-19.
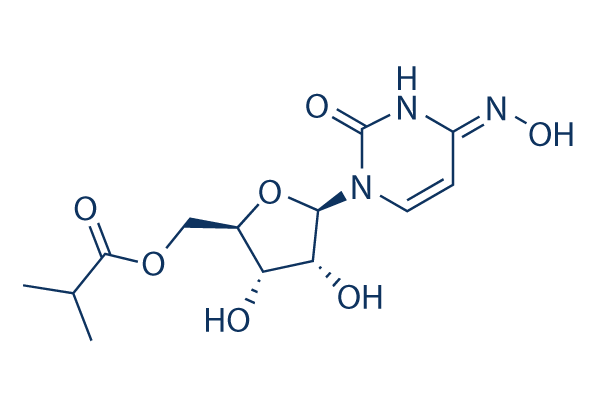
Wie es das Coronavirus stoppt war bisher unklar. If authorised by regulatory bodies molnupiravir could be the first oral antiviral medicine for Covid. Molnupiravir ist das Prodrug des synthetischen Nukleosid derivats N4-Hydroxycytidin NHC.
Once that process is underway the. Antiviral treatments now in use such as remdesivir are administered intravenously CNBC reported. Its administered as a twice-a-day pill for five days compared to other Covid-19 treatments that.
Die Firmen MSD und Ridgeback Biotherapeutics haben nach einer Zwischenanalyse angekündigt eine Phase-III-Studie bei nicht hospitalisierten Covid-19-Patienten fortzusetzen. Merck Says Experimental Pill Molnupiravir Cuts Worst Effects Of COVID-19. In a trial of 775 patients with mild-to-moderate COVID-19 who were considered higher risk for severe disease molnupiravir reduced hospitalization by 50.
Der antivirale Wirkstoff Molnupiravir ist derzeit als mögliches Covid-19-Medikament im Gespräch. Molnupiravir an Oral Antiviral Treatment for COVID-19. Molnupiravir is the first oral direct-acting antiviral shown to be highly effective at reducing nasopharyngeal SARS-CoV-2 infectious virus and viral RNA and has a favorable safety and tolerability profile.
Derzeit 2021 wird in klinischen Studien ein möglicher Einsatz bei COVID-19-Patienten geprüft. Das Medikament Molnupiravir der US-Firma MSD senkt offenbar das Risiko für Krankenhausaufenthalt und Tod durch Covid-19 um die Hälfte. Erst der aktive Metabolit kann in die virale RNA eingebaut werden um einen Kopierfehler zu.
Meet molnupiravir Mercks Thor-inspired pill that hammers COVID In Phase III trial the drug smashed hospitalization and death rate by about half. Es soll bei Covid-19-Patienten eingesetzt werden die seit fünf oder weniger Tagen Symptome haben und ein. Molnupiravir MK-4482 is designed to induce viral genome copying errors to prevent the virus from replicating in the human body and evidence to date from clinical trials in patients with COVID-19 suggests that molnupiravir may reduce replication of the SAR-CoV-2 virus.
Molnupiravir a wide-spectrum antiviral that is currently in phase 23 clinical trials for the treatment of COVID-19 is proposed to inhibit viral replication by a. Merck Molnupiravir EIDD-2801MK-4482 is an investigational orally bioavailable form of a potent ribonucleoside analog in development for the treatment of COVID-19. An effective antiviral therapeutic has since been intensively sought.
The drug looks enough like some of the natural building blocks that the. Jetzt haben Göttinger. This treatment is being evaluated in an ongoing Phase 3 trial for its potential to reduce the risk.
Eigentlich sollte es ein Mittel gegen Grippe werden. WASHINGTON AP Drugmaker Merck said Friday that its experimental COVID-19 pill reduced hospitalizations and deaths by. Tatsächlich zeigt sich Molnupiravir wirksam gegen Sars-CoV-2.
Molnupiravir works by confusing SARS-CoV-2s polymerase the enzyme that builds the viral genome during replication. Molnupiravir originally developed to treat influenza could solve many of these challenges. Merck which is co-developing the drug with Ridgeback says it plans to.
Molnupiravir if approved would be the first orally active direct-acting antiviral drug for COVID a significant advance in fighting the pandemic. Ad Apotal Onlineapotheke - günstiger gehts kaum. Molnupiravir demonstrated activity in preclinical models of SARS-CoV-2 SARS-CoV-1 and MERS including prophylaxis cure and transmission prevention.
As of June 25 2021 SARS-CoV-2 has infected over. Bei Molnupiravir handelt es sich um ein Prodrug des synthetischen Nukleosidderivates N4-Hydroxycytidin. Mit der Smart-TV App holen Sie sich Ihre Apotheke ins Wohnzimmer.
Molnupiravir an oral antiviral treatment for COVID-19. Molnupiravir originally created by researchers at Emory University in Atlanta is given as four pills taken twice a day for five days. Molnupiravir an Oral Antiviral Treatment for COVID-19 medRxiv.
Molnupiravir wird von Merck in Zusammenarbeit mit Ridgeback Biotherapeutics entwickelt. Last updated by Judith Stewart BPharm on July 14 2021. Molnupiravir tricks the coronavirus into using the drug to try to replicate the viruss genetic material.

Wie Das Potenzielle Coronamedikament Molnupiravir Wirkt
Molnupiravir Eidd 2801 99 Hplc Selleck Sars Cov Inhibitor

Molecular Mechanisms Of Corona Drug Candidate Molnupiravir Unraveled Max Planck Gesellschaft

Neue Studiendaten Molnupiravir Schutzt Mause Vor Corona Infektion Und Cov Pz Pharmazeutische Zeitung
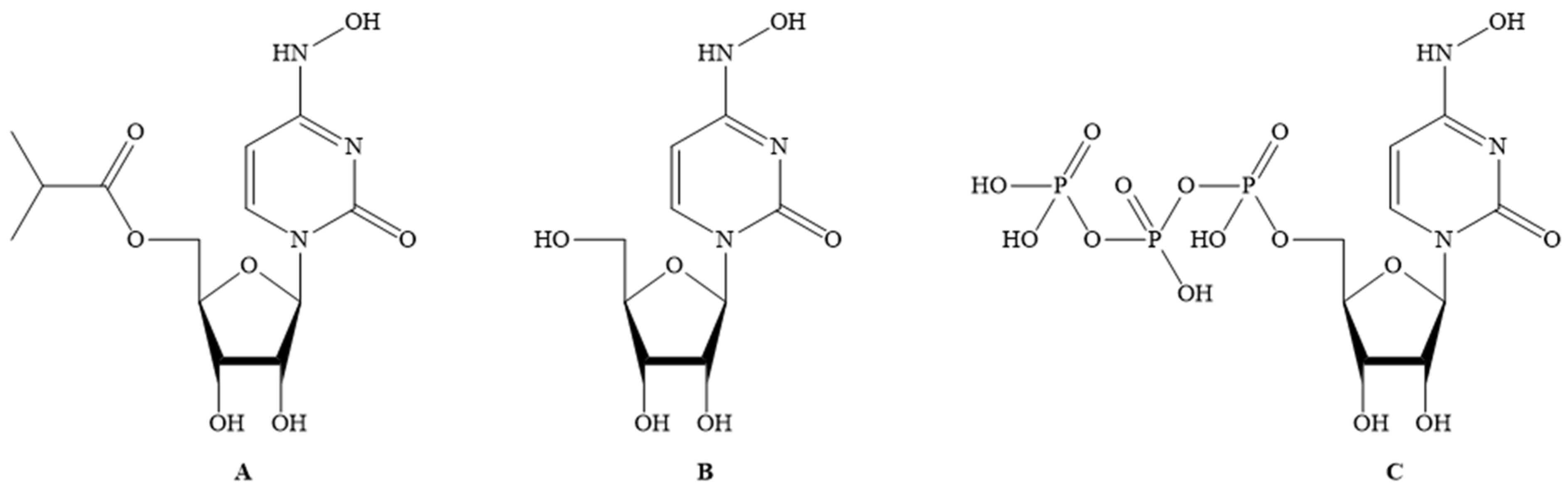
Molecules Free Full Text Discovery Development And Patent Trends On Molnupiravir A Prospective Oral Treatment For Covid 19

A Daily Pill To Treat Covid Could Be Just Months Away Scientists Say

Weniger Schwere Krankheitsverlaufe Corona Medikament Weckt Hoffnung Tagesschau De